From Your Regulatory Compliance Specialist-Linda Cannon: Covid-19: #15, VDA Reminds Members of…
|
|
|
NATIONAL WELLNESS MONTH – August
All during August, National Wellness Month focuses on self-care, managing stress, and promoting healthy routines. Create wholesome habits in your lifestyle all month long and see how much better you feel!
Research has shown self-care helps manage stress and promotes happiness. Whether you challenge yourself to a new yoga pose or try a different spa treatment, make a small change and impact your health in positive ways. |
There are numerous ways to make those small changes, too.
Increase your water intake.
Add more fruits and veggies to your meals.
Monitor your sleep and make adjustments for better sleep habits
Join a yoga, walking, or aerobics class.
Learn to meditate.
These small steps can lead to many more healthy habits in your lifestyle.
HOW TO OBSERVE #WellnessMonth #Wellfie
Proclaim “I choose wellness” with a photo and post on social media.
Every August, let’s amplify the message of healthy living, celebrate those providing amazing self-care solutions, and inspire others to create new healthy habits.
Visit www.wellnessmonth.com for a calendar of daily challenges for small ways you can choose to be well each day. Because we believe that small, daily acts of self-care lead to a lifetime of wellness.
For more ways to add wellness to your daily life follow National Wellness Month:
#wellnessmonth #wellfie #ichoosewellness
www.instagram.com/wellness_month
www.facebook.com/wellnessmonth
Twitter: @wellness_month
Pinterest.com/wellnessmonth
NATIONAL WELLNESS MONTH HISTORY
Health: FDA adds new warning on Johnson & Johnson vaccine
FDA adds new warning on Johnson & Johnson vaccine related to rare autoimmune disorder
The agency cites link to a small number of Guillain-Barré cases after vaccination but says the shot’s benefits outweigh the risk
The Food and Drug Administration announced a new warning for the Johnson & Johnson coronavirus vaccine on July 12. (Reuters)
By
Laurie McGinley
and Lena H. Sun
July 12, 2021|Updated yesterday at 11:42 a.m. EDT
The Food and Drug Administration announced a new warning for the Johnson & Johnson coronavirus vaccine on Monday, saying the shot has been linked to a serious but rare side effect called Guillain-Barré syndrome, in which the immune system attacks the nerves.
About 100 preliminary reports of Guillain-Barré have been detected in vaccine recipients after the administration of 12.8 million doses of the Johnson & Johnson vaccine in the United States, according to a companion statement from the Centers for Disease Control and Prevention, which monitors vaccine safety systems with the FDA. Of these reports, 95 were serious and required hospitalization, the FDA statement said. There was one death. Neither agency provided details about the death.
The cases have largely been reported about two weeks after vaccination and mostly in men, many aged 50 and older, according to the CDC. Most people fully recover from Guillain-Barré.
The warning on the Johnson & Johnson vaccine will be the latest blow to a shot that had been eagerly anticipated because of its ease of use — it requires only a single dose, which makes it especially helpful in immunizing harder-to-reach populations and regions. But the vaccine has been plagued by problems, including massive stumbles at its U.S. manufacturing plant.
The latest development comes at an especially fraught moment, as the highly transmissible delta variant sweeps the country and fuels an increase in coronavirus cases in many states. And the new warning could complicate the Biden administration’s efforts to ramp up inoculations in areas where skepticism regarding coronavirus vaccines remains high and the vaccine rate is low.
Available data do not show a pattern suggesting a similar increased risk of Guillain-Barré with the Pfizer-BioNTech and Moderna vaccines. More than 321 million doses of those vaccines — developed with a technology different from what is used to make the Johnson & Johnson vaccine — have been administered in the United States.
The Guillain-Barré cases are expected to be discussed as part of next Thursday’s meeting of the CDC’s Advisory Committee on Immunization Practices, the agency said.
The Johnson & Johnson coronavirus vaccine is a single shot, making it especially useful in areas where it might be harder to administer two shots. (Narendra Shrestha/EPA-EFE/Shutterstock)
Although the available evidence suggests an association between the Johnson & Johnson vaccine and increased risk of Guillain-Barré, “it is insufficient to establish a causal relationship,” the FDA said.
Guillain-Barré syndrome usually occurs at a rate of about 60 to 120 cases each week, according to CDC data. While the cause is not fully understood, it often follows infection with a virus, including influenza, or bacteria. Each year in the United States, an estimated 3,000 to 6,000 people develop the illness.
People older than 50 are at greatest risk. About two-thirds of people who develop the syndrome experience symptoms several days or weeks after they have fallen ill with diarrhea or a lung or sinus illness.
Federal health officials have repeatedly emphasized that the benefits of the coronavirus inoculations far outweigh potential risks. The FDA said it had evaluated the available information for the Johnson & Johnson vaccine “and continues to find the known and potential benefits clearly outweigh the known and potential risks,” the agency said.
Johnson & Johnson declined Monday to comment.
Reports of Guillain-Barré in vaccine recipients are rare, the CDC said, “but do likely indicate a small possible risk of this side effect following” the Johnson & Johnson vaccine. Reports of the syndrome were made to the Vaccine Adverse Events Reporting System, an early warning safety network run by the CDC and the FDA. It collects information about possible side effects or health problems after vaccination. The system looks for unusual or unexpected patterns that require a closer look. Anyone can report a reaction or injury.
Experts said the latest news about the Johnson & Johnson shot may complicate vaccination efforts, especially in parts of the country where rates remain under 50 percent.
Jeanne Marrazzo, director of infectious-diseases at the University of Alabama at Birmingham, said the absolute risk of Guillain-Barré remains so rare that “it should not deter people from getting vaccinated,” she wrote in an email. If people are concerned, they can still get a Pfizer or Moderna vaccine with no risk, adding: “This news does not provide an excuse to remain unvaccinated!!!!”
But the expected warning on the Johnson & Johnson shot “quite possibly” will make it harder for health-care providers to persuade people to roll up their sleeves, Marrazzo said.
“When a person is hesitant to get a vaccine, any additional safety signal, even if very rare, just adds to their own database that fuels their reluctance,” Marrazzo wrote.
Paul A. Offit, a pediatrician and director of the Vaccine Education Center at Children’s Hospital of Pennsylvania, said he did not think the latest news will make much difference because the U.S. vaccination effort has “hit a wall.” The Biden administration has already done “as good a job as we could possibly hope for on how to mass-produce, mass distribute and mass vaccinate” the country, Offit said.
Now, Offit said, it’s time for the next step: the government needs to compel vaccination. The Supreme Court has already ruled twice that public health authorities can take these measures in the face of outbreaks, he said. Without such a step, the virus is “going to continue to mutate, continue to create variants” and result in another surge in late fall and winter, he said.
Just three months ago, federal officials paused use of the Johnson & Johnson vaccine after it was linked to another rare side effect — severe blood clots. That pause was lifted within days after an extensive safety review by the FDA and the CDC, and a warning was added to the vaccine’s label.
The vaccine also has been hobbled by production problems at Johnson & Johnson’s subcontractor, Emergent BioSolutions, the only U.S. manufacturer of the vaccine. The Baltimore plant was shut down in April after federal officials discovered millions of doses had been contaminated with the AstraZeneca vaccine, which was also being made there.
Johnson & Johnson had to throw away the equivalent of about 75 million doses of the vaccine because of the problems at the Baltimore plant. About 40 million doses have been released for use. In response to the contamination, the Biden administration removed AstraZeneca manufacturing from the plant and put Johnson & Johnson in direct control of vaccine production there. But Emergent has not received authorization from the FDA to resume manufacturing the Johnson & Johnson product.
Other vaccines also have been associated with rare adverse events. The FDA in late June decided to add a warning to the Pfizer and Moderna coronavirus vaccines about extremely unusual cases of myocarditis — heart inflammation — in some young adults and teens after vaccination. Federal health officials said there was “a likely association,” and that the problem appears most likely to occur in young men after they receive two doses of the vaccine.
The CDC and the Department of Health and Human Services, together with 15 of the country’s leading medical and public health organizations, issued a joint statement in June saying they “strongly encourage everyone 12 and older” to get the Pfizer and Moderna shots because the benefit of vaccination far exceeds potential harm.
In June, the American Neurological Association reported that two studies published in the journal Annals of Neurology had found 11 cases of Guillain-Barré syndrome two to three weeks after vaccination with the AstraZeneca vaccine. The cases, which were from England and India, involved an unusual variant of the disease that caused severe facial weakness, the organization said. An accompanying editorial described a similar case involving a Boston man who received the Johnson & Johnson vaccine.
Vaccine safety officials in Europe have recommended that a warning be added about Guillain-Barré to the AstraZeneca vaccine. But the European safety committee said that while cases have been reported following vaccinations, “at this stage the available data neither confirms nor rules out a possible association with the vaccine.”
The FDA revised its Johnson & Johnson fact sheet, urging vaccine recipients to seek immediate medical attention if they develop weakness or tingling, especially in the legs or arms, that worsens and spreads to other parts of the body; difficulty walking; difficulty with facial movements, including speaking, chewing or swallowing; double vision or inability to move the eyes; or difficulty with bladder control or bowel function.
In 1976, there was a small increased risk of Guillain-Barré syndrome after people received the swine flu vaccine, which was a special shot for a potential pandemic strain of the influenza virus. A National Academy of Medicine review in 2003 found that people who received the 1976 swine flu vaccine had an increased risk of Guillain-Barré, with about one additional case for every 100,000 people who received the swine flu vaccine. The reason for the link remains unknown.
The CDC monitors for Guillain-Barré syndrome each flu season. The agency says the data on an association between the seasonal influenza vaccine and the illness varied from season to season. When there has been an increased risk, it has consistently been in the range of one or two additional cases per 1 million flu vaccine doses administered. Studies also suggest it is more likely that a person will get Guillain-Barré after getting the flu than after vaccination, according to the CDC.
Read more:
Pfizer expected to brief Biden administration officials on need for booster shots
Drop in childhood vaccinations during pandemic may raise risk of other outbreaks when schools reopen, CDC says Coronavirus vaccines are widely available in the U.S. So why are scientists working on new ones? Updated June 23, 2021
Coronavirus: What you need to read
Coronavirus maps: Cases and deaths in the U.S. | Cases and deaths worldwide
Vaccines: Tracker by state | Guidance for vaccinated people | Kids | How long does immunity last? | County-level vaccine data
What you need to know: Delta variant | Other variants | Symptoms guide | Masks FAQ | Personal finance guide | Follow all of our coverage and sign up for our free newsletter
Got a pandemic question? We answer one every day in our coronavirus newsletter
https://www.aha.org/news/headline/2021-07-13-fda-adds-new-warning-jj-covid-19-vaccine?fbclid=IwAR03CpJxzHDG6BkDy_9l0Q6-crH7vyCM7GmT6ljVXzNenwfhlz1QhNMtPag
ADA talks Federal Regulations, including Video.
Washington — The ADA is pleased that dental practices are mostly exempt from the Occupational Safety and Health Administration’s new emergency temporary standard to protect health care workers from COVID-19.
The OSHA emergency standard, released June 10, provides additional guidance for health care settings, including hospitals and nursing homes, where all employees may not be screened for COVID-19, and non-employees and patients with suspected or confirmed cases of COVID-19 are allowed to enter and may be treated. Dental offices most likely to be affected by the new standard include hospital-based oral surgery practices or those dentists who provide care for COVID-19 patients.
“The great news is that dentistry is largely exempt from this additional federal regulation,” said ADA President Daniel J. Klemmedson, D.D.S., M.D., in a video message to dentists. “Why? Because of dentistry’s proven ability to practice safely during the pandemic.”
Dr. Klemmedson pointed to the profession’s widespread adoption of the guidance outlined in the ADA Return to Work Interim Guidance Toolkit as key to that success and praised his fellow dentists for their strong adherence to the use of increased personal protective equipment and infection control precautions throughout the pandemic.
“The new infection control guidance and very low COVID-19 infection rate for dentists and dental hygienists prove that dental practices are safe workplaces,” said Dr. Klemmedson in an ADA news release.
Dental offices should have a written COVID-19 plan in place. If an office is covered under the emergency temporary standard, it is mandated to do so. If an office is exempt, it still should do a hazard assessment and written plan as recommended in OSHA’s Recommended Practices for Safety and Health Programs. Dental practices must also conduct workplace-specific hazard assessments for COVID-19 and should also continue pre-appointment patient screenings to identify individuals with suspected or confirmed COVID-19, rescheduling their appointments if possible or referring them as necessary.
The ADA has been advocating for dentistry on this issue even before January, when President Joe Biden issued an executive order making the health and safety of workers a priority. Earlier this month, Dr. Klemmedson and ADA staff met with the White House Office of Management and Budget to discuss how the rule could impact dentistry. During that meeting, the Association told officials there wasn’t a “grave danger of being exposed to COVID-19 in dental settings, particularly as the pandemic is decelerating” and noted that dentists have experienced “exceptionally low monthly incidences of COVID-19” despite several regional and national spikes during OSHA’s study periods.
“This is a win for science, a win for ADA advocacy and especially a win for you,” Dr. Klemmedson told dentists in his video message. “Be well.”
To help dentists understand the OSHA emergency temporary standard, the ADA has created a fact sheet that includes some key points to help walk dentists through the process. Visit ADA.org/virus to access the fact sheet.
Follow all of the ADA’s advocacy efforts at ADA.org/Advocacy
ADA President’s Update: Dentistry Largely Exempted from New Federal COVID Regulation
https://www.youtube.com/watch?v=69t2Esk8ayc
VDA reminds Virginia Dentist – OSHA Federal Regulations Are stricter than what the ADA stated in Video. Please read!!  Policy and Regulatory Changes This Week that Impact Virginia Dentists State of Emergency Ends, DOLI FPS Still in Place Policy and Regulatory Changes This Week that Impact Virginia Dentists State of Emergency Ends, DOLI FPS Still in PlaceAs you are aware, Governor Northam’s Executive Order expired yesterday, ending the state of emergency in Virginia that was first declared on March 12, 2020. However, the end of the state of emergency does not signify the end of the Virginia Department of Labor and Industry (DOLI) Full Permanent Standard (FPS) which governs workplace standards. For healthcare settings such as dental offices, this means that masks must still be worn by all personnel, regardless of vaccination status. In non-healthcare settings, masks must be worn by all unvaccinated employees. Please be sure to refer to the DOLI FPS to ensure that your practice remains in compliance with all standards. The current DOLI FPS is under review and a set of revisions approved by the Safety and Health Codes Board yesterday are undergoing executive review by the Governor’s Office. The VDA will work to keep all members informed of any changes to the FPS. Virginia’s Adult Medicaid Dental Benefit Virginia’s adult Medicaid Dental Benefit goes into effect July 1, providing new benefits to about 750,000 Virginians. The new coverage includes preventative and diagnostic treatment, such as X-rays and exams, and oral surgery and prosthodontics, which includes items like dentures. Find provider resources and patient fact sheets in English and Spanish from DMAS. The VDA has additional resources available including a Q&A with Dr. Zachary Hairston. e-Prescribe Waivers Expire on July 1 If you received a waiver from the Virginia Board of Dentistry temporarily delaying electronic prescribing of opioids, it expires today. VDA Members can receive 43% off e-Prescribe solutions from iCoreRx. Visit iCore online or call 888.810.7706 to book your no-obligation demo. Please note that this information is provided as a member benefit and is not intended to constitute legal advice. Each member is encouraged to consult their counsel for guidance on all issues including liability. |
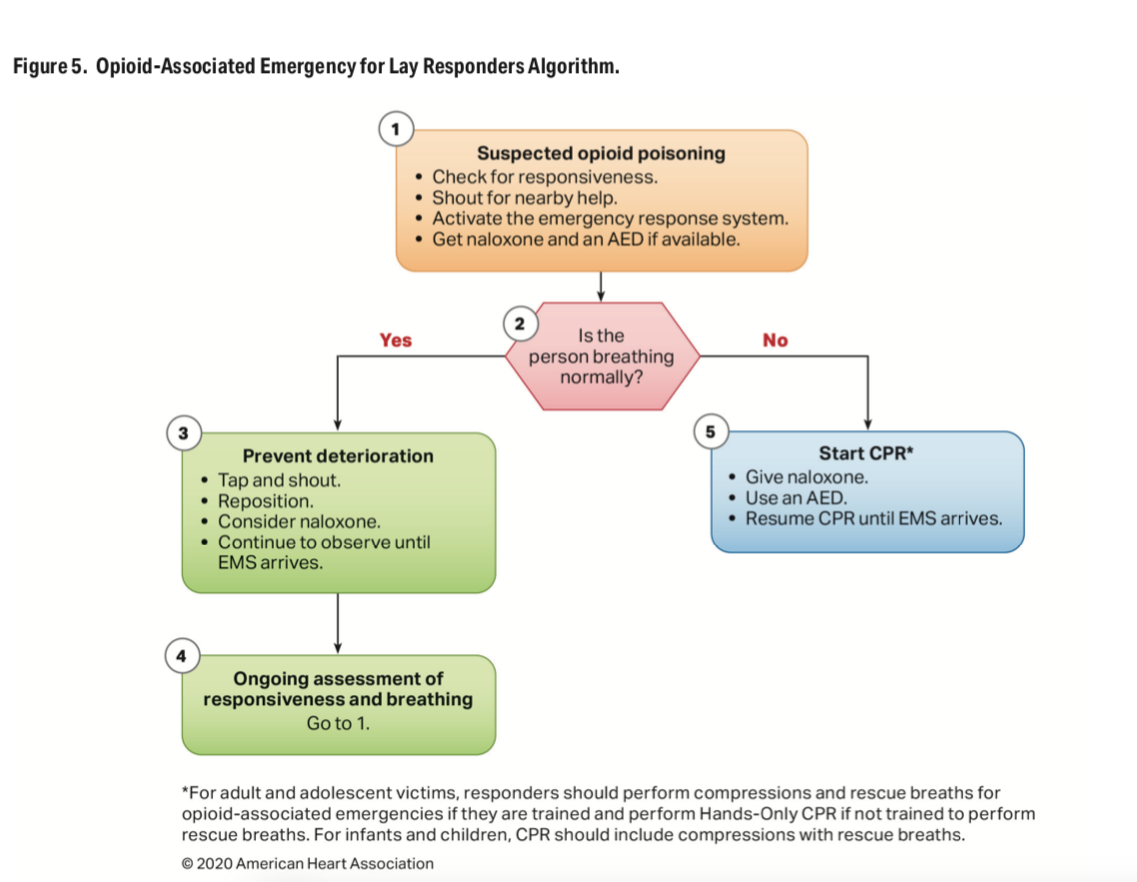
| American Heart Association updates CPR guidelines: 5 key changes The American Heart Association updated 491 recommendations for adult, pediatric and neonatal life support to increase cardiac arrest survival rates, according to a summary of its 2020 guidelines released Oct. 21. Five key updates: Cardiac arrest survivors should have a comprehensive recovery phase. This includes a formal assessment after the initial hospitalization to support patients’ “physical, cognitive, and psychosocial needs,” according to the summary. This support should continue as long as needed after the initial hospitalization. Lay rescuers, or bystanders, should initiate CPR early on. The AHA said the risk of harm from CPR is low, even if the patient is not in cardiac arrest. Pediatric resuscitation increased to one breath every two to three seconds. This provides 20-30 breaths per minute. Naloxone should be administered for cardiac arrest caused by an opioid overdose. CPR should also be given immediately. Fetal heart monitoring is not helpful during maternal cardiac arrest. Attempting to evaluate the fetal heart may distract from maternal resuscitation. The addition of a recovery phase is one of the biggest changes to AHA guidelines, Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee who helped write the guidelines and associate professor of emergency medicine at the University of Pennsylvania in Philadelphia, told American Heart Association News. “It’s a new focus on what we can do to support their physical, cognitive, and psychosocial needs after they have a cardiac arrest and after they leave the hospital,” Dr. Merchant said. “The latest research tells us that’s really important.” |
CDC recommends masks for vaccinated people in high transmission areas.
The Centers for Disease Control and Prevention recommends that even vaccinated Americans resume wearing masks indoors if they are in areas with high or substantial transmissibility of the COVID-19 virus.
The guidance also recommends for people with underlying conditions wear masks, along with anyone living with vulnerable people. Teachers, school staff, students and visitors inside schools from kindergarten to 12th grade also fall under new guidance recommending universal mask-wearing.
For vaccinated people in just under half of the counties in the country – those with high transmissibility – that means masks are recommended indoors once again.
Several counties with major U.S. urban centers, including Los Angeles, St. Louis, and Miami-Dade, are among the high transmission counties.
Mapping CDC’s new guidelines: High transmission areas where you need to wear a mask indoors
“I thought I did everything right”:
The fully vaccinated are frustrated by CDC’s changing mask advice
Here’s what transmissibility is and how to find out if you’re in an area where you should start masking indoors again:
Map of high COVID-19 transmission areas
The CDC provides a COVID-19 data tracker that includes a by-county view of transmissibility rates each week. Check out the map below. You can also visit the CDC’s website to enter your state, county, or metro area to find out what transmissibility is like where you live. 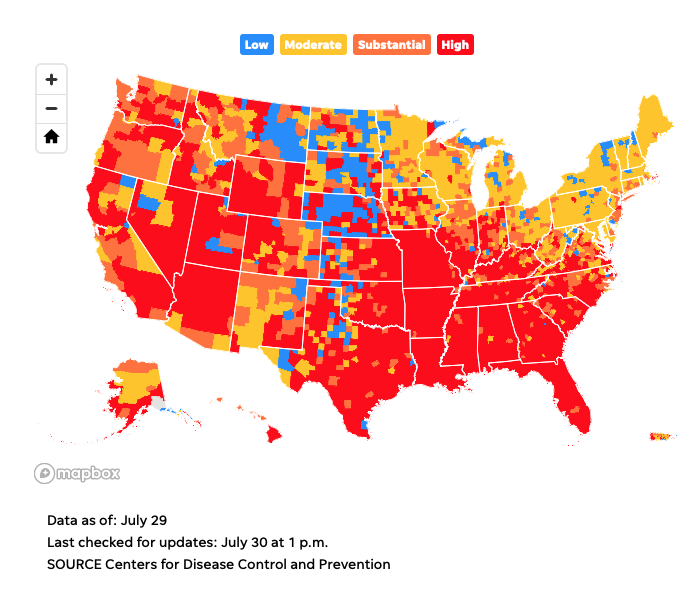 https://www.usatoday.com/story/news/nation/2021/07/27/cdc-recommends-masks-high-transmissibility-areas-what-does-mean/5394057001/
https://www.usatoday.com/story/news/nation/2021/07/27/cdc-recommends-masks-high-transmissibility-areas-what-does-mean/5394057001/
| WINNER OF THE WEEK |
Winner of the week Instead of the “Question of the week”; MSDS will be adding: *Questions and concerns from our clients *Literature of Potential scam/spam *Updates from the Board of Dentistry *New Regulations from Federal or State *And Grammar/Spelling Errors.  Weekly winners will receive a $10.00 gift card to Starbucks!Names are drawn by the app below. https://miniwebtool.com/random-name-picker/  |
| Organizing your computer for MSDS: Make a folder on your desktop named MSDS. Double click the folder. Create Folders for each category. OSHA-BBP, OSHA Safety Classes, HIPAA, Medical Emergency, BLS-CPR, etc. In each folder, for example, HIPAA: You’ll have the Business Associate Agreement, Patient Consent Form, Office’s Privacy Practice, etc. |









