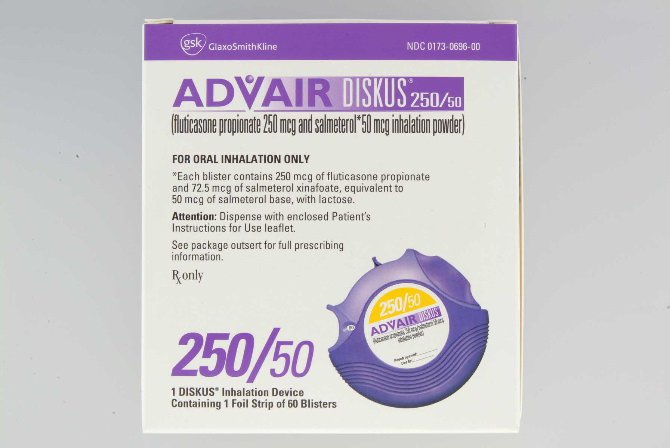
Description
Product Specifications
MSDS # m626728
Manufacturer # 00173069600
Brand Advair Diskus®
Manufacturer Glaxo Smith Kline
Country of Origin Unknown
Alternate Manufacturer Number 1284538
Application Corticosteroid / Beta-Adrenergic Agonist
Container Type Metered-Dose Inhaler
Dosage Form Powder
Generic Drug Code 50594
Generic Drug Name Fluticasone / Salmeterol
NDC Number 00173069600
Product Dating McKesson Acceptable Dating: we will ship >= 90 days
Quantity 60 Doses
Strength 250 mcg – 50 mcg
Type Inhalation
UNSPSC Code 51162030







Reviews
There are no reviews yet.