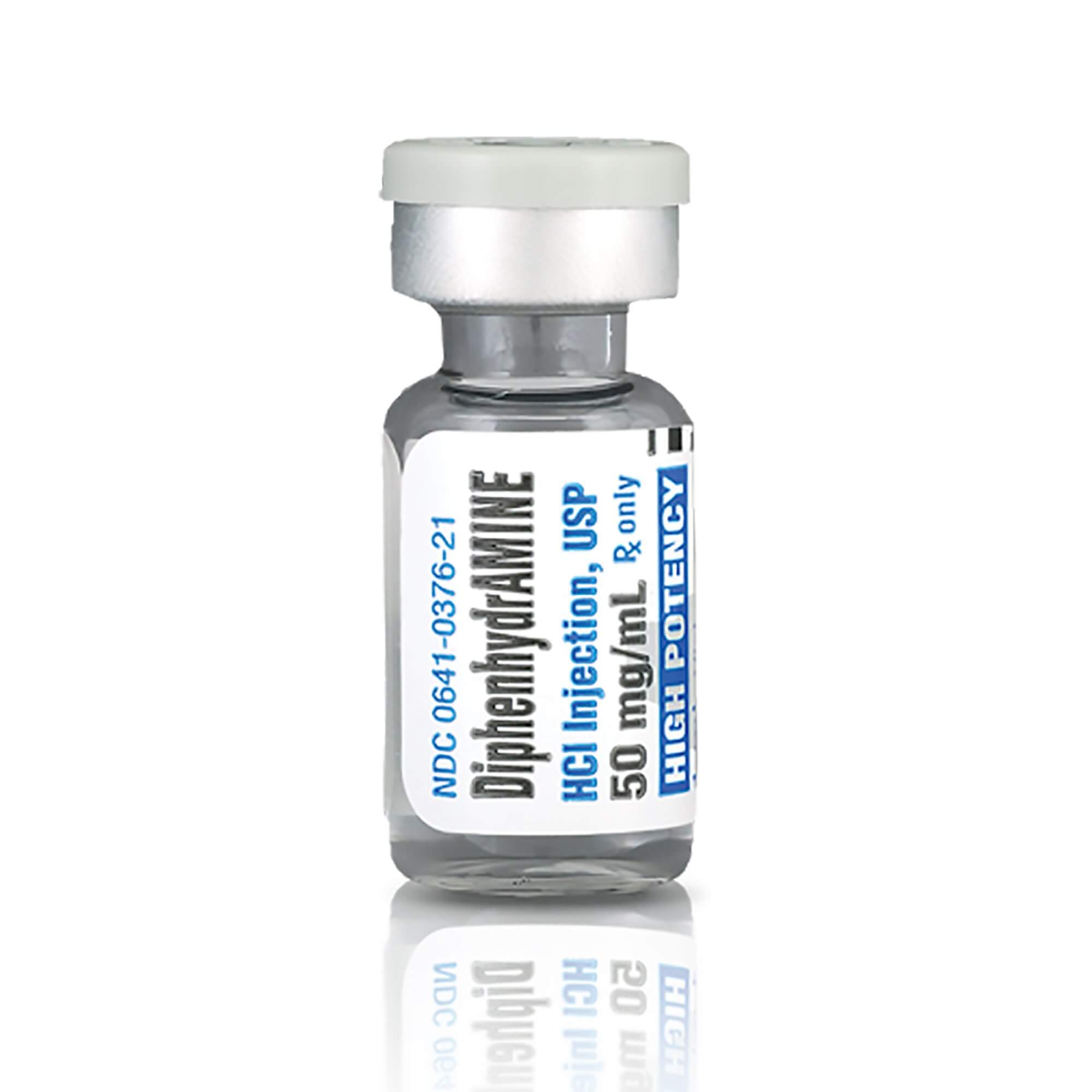
Description
Product Specifications
MSDS # M246042
Manufacturer # 00641037625
Manufacturer Hikma Pharmaceuticals USA
Country of Origin United States
Alternate Manufacturer Number 2283521
Alternate Packaging BX/25
Application Antihistamine
Container Type Vial
Dosage Form Injection
Generic Drug Code 46013
Generic Drug Name Diphenhydramine HCl
NDC Number 00641037625
Product Dating McKesson Acceptable Dating: we will ship >= 90 days
Strength 50 mg / mL
Type Intramuscular or Intravenous
UNSPSC Code 51312309
Volume 1 mL






Reviews
There are no reviews yet.